What does it mean to mix an s and p orbital?
- As you can see on the diagram, s and p orbitals are at different energy levels.
- However, the s and p orbitals can move to a energy level in between both of them. This is known as hybridisation.
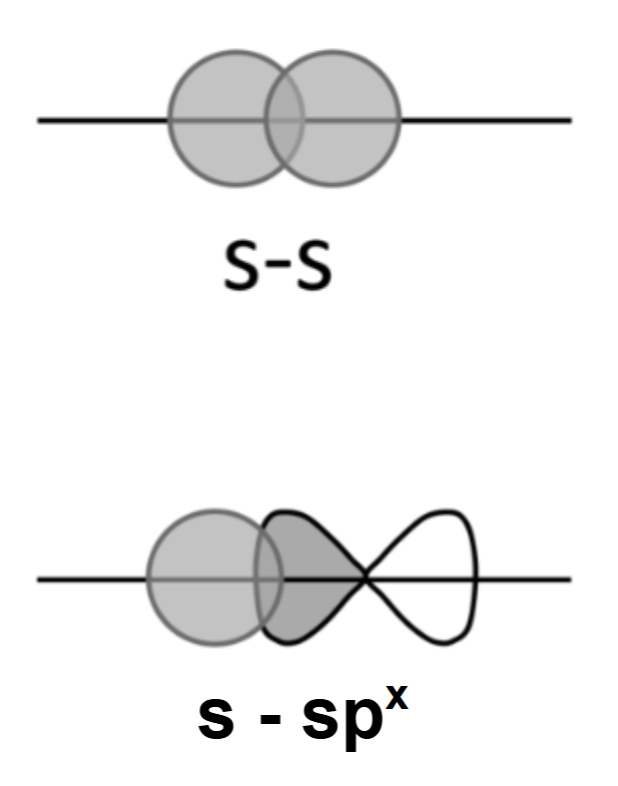
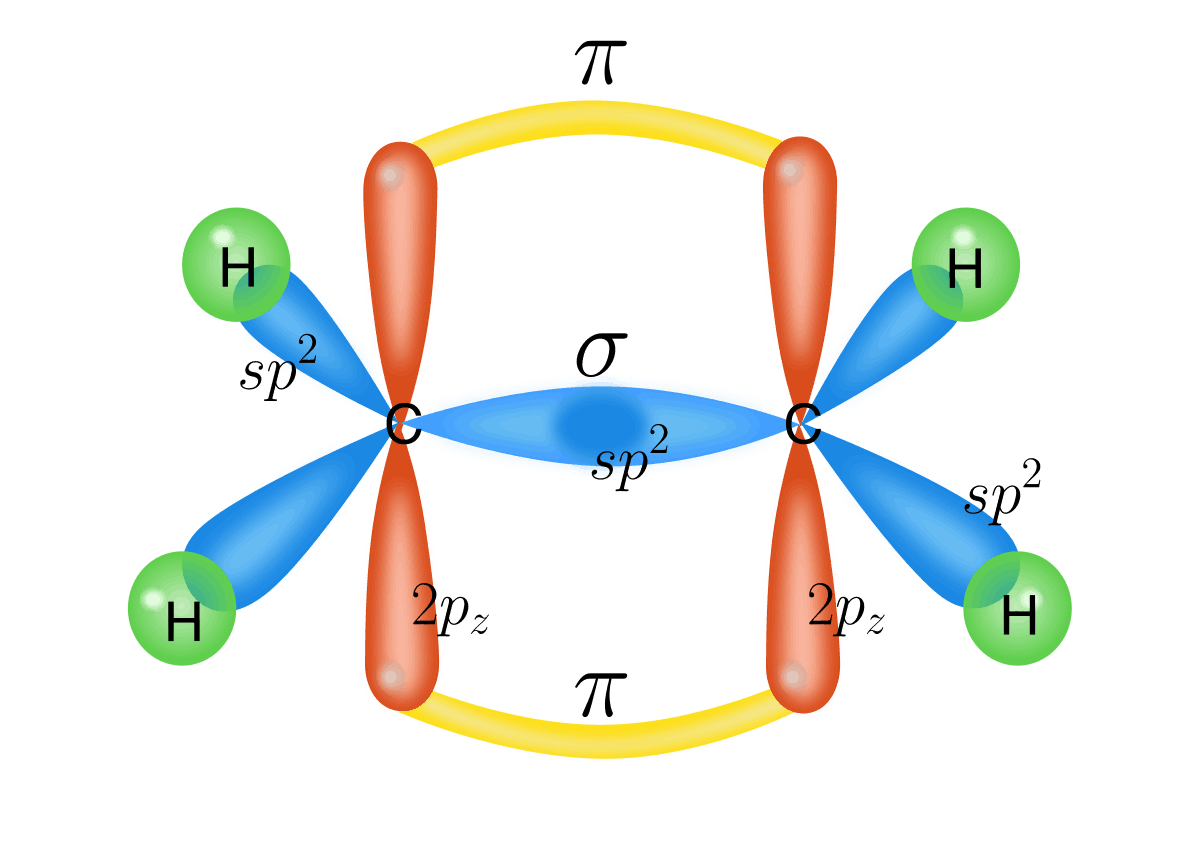
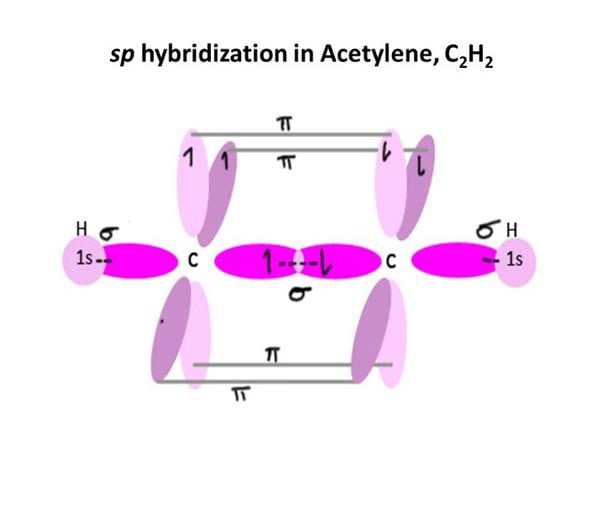
- s, sp1, sp2 and sp3 orbitals can only overlap end-on-end to form σ bonds whereas p orbitals can overlap sideways to form \( \pi \) bonds.
- The first bond must always be a σ bond but the second and third bond must be a \( \pi \) bond.