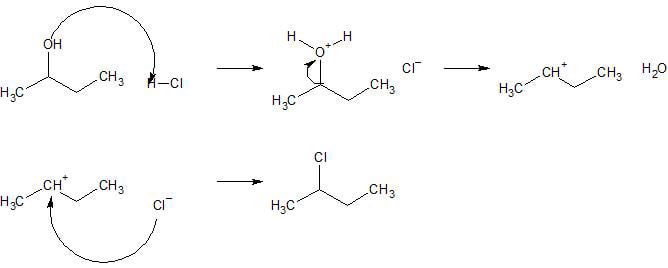
SN1 reaction
- Occurs with tertiary halogenoalkanes. In these, the carbon atom bonded to the halogen atom is also bonded to three other carbon atoms (alkyl groups).
- Mechanism:
1. The carbon–halogen bond breaks, forming a tertiary carbocation. This is an example of heterolytic fission as both electrons in the bond go to the halogen.
2. The tertiary carbocation is immediately attacked by the hydroxide ion. - The rate of the reaction only depends on the concentration of the halogenoalkane only, as shown in the first (slow) step of the mechanism.
rate = k[halogenoalkane]