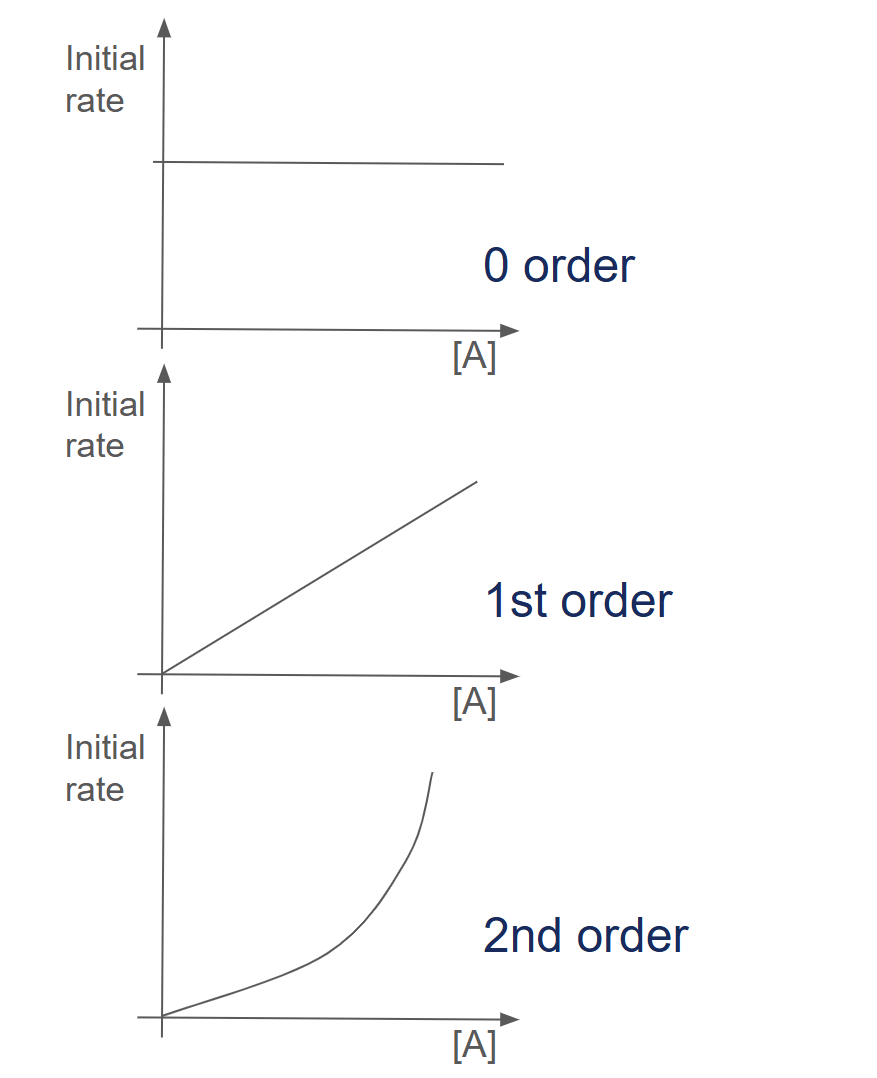
Method 1: Using rate against concentration graphs
- Let's say that we are trying to find the order of A in the reaction A + B → C. We will carry out many different experiments with different concentrations of A and an excess of B. We then plot a graph of the initial rate of reaction against concentration of A.
- For a zero-order reaction, the plot of reaction rate against concentration is a horizontal straight line. The reaction rate does not change with concentration.
- For a first-order reaction, the plot is an inclined straight line going through the origin. The rate is directly proportional to the concentration.
- For a second-order reaction, the plot is an upwardly curved line. The reaction rate is directly proportional to the square of the concentration.