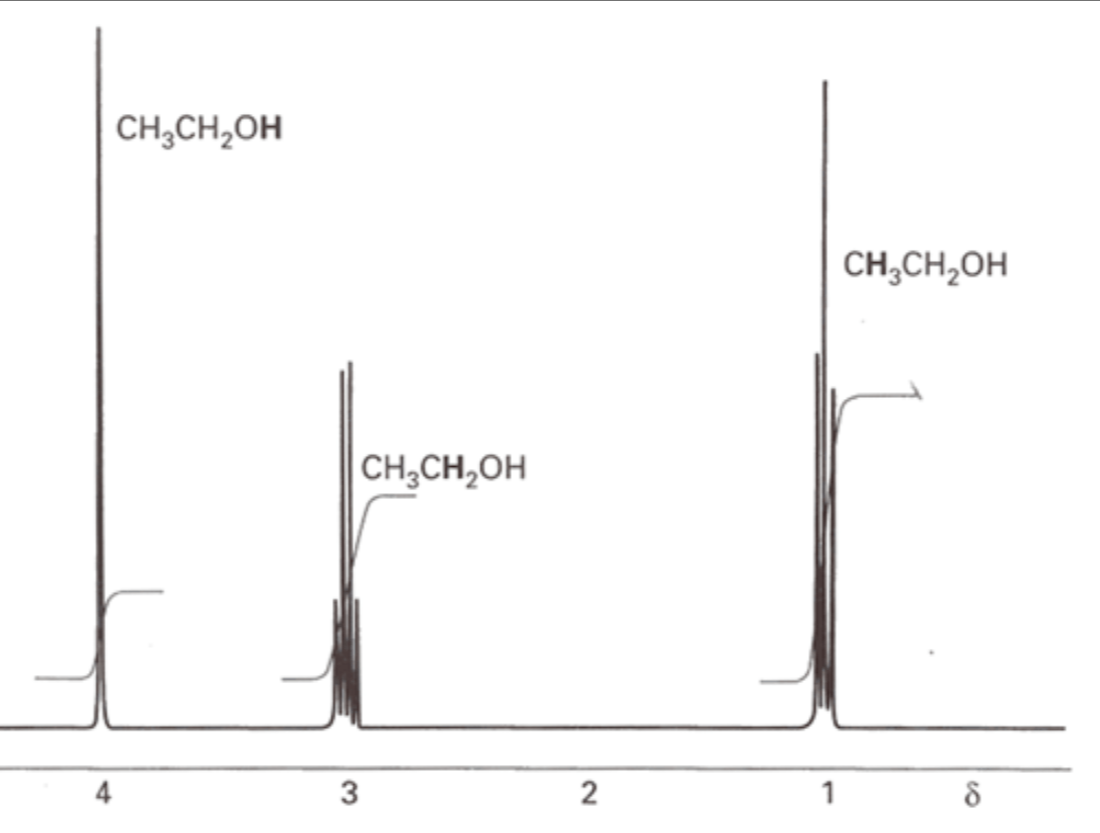
The output of an H-NMR looks like this.
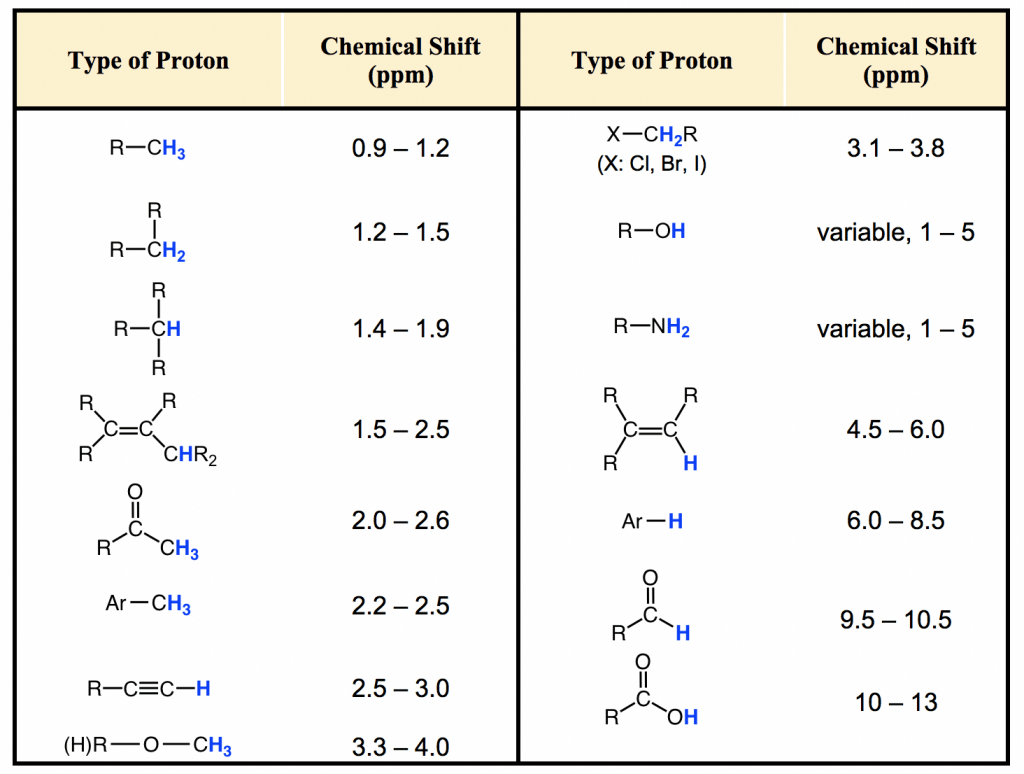
We must pay attention to the chemical shift of each peak and the number of peaks.
The ppm value (chemical shift) is used to determine what the proton is bonded to by comparing it with a graph of known values.
The peaks tell us how many H atoms there are on surrounding carbon atoms. The number of H atoms is given by the equation: number of peaks - 1.