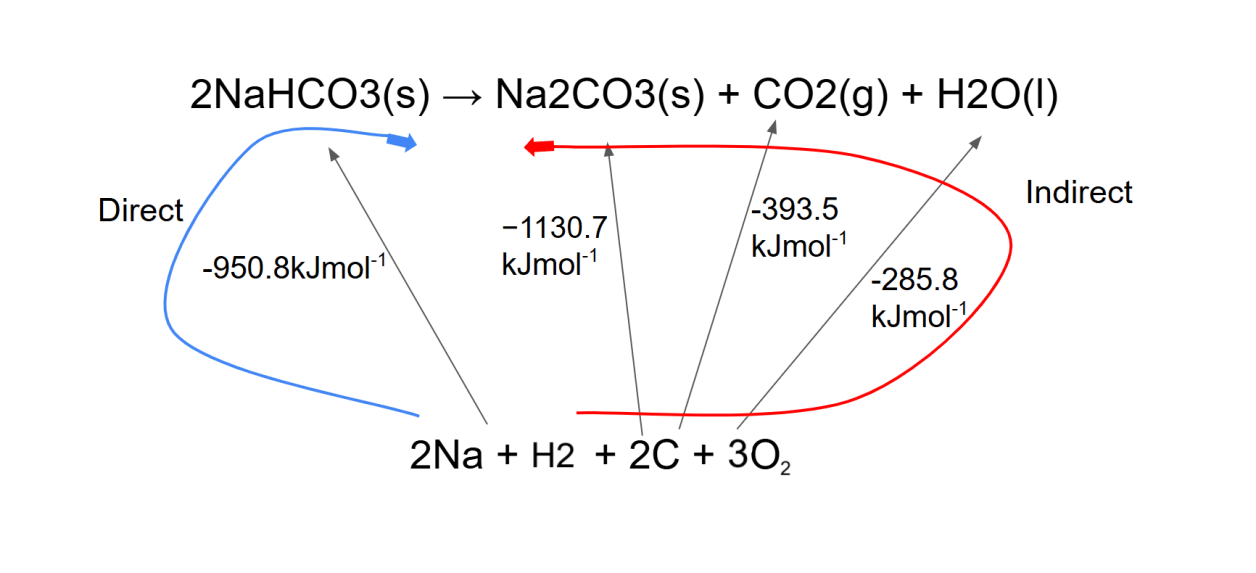
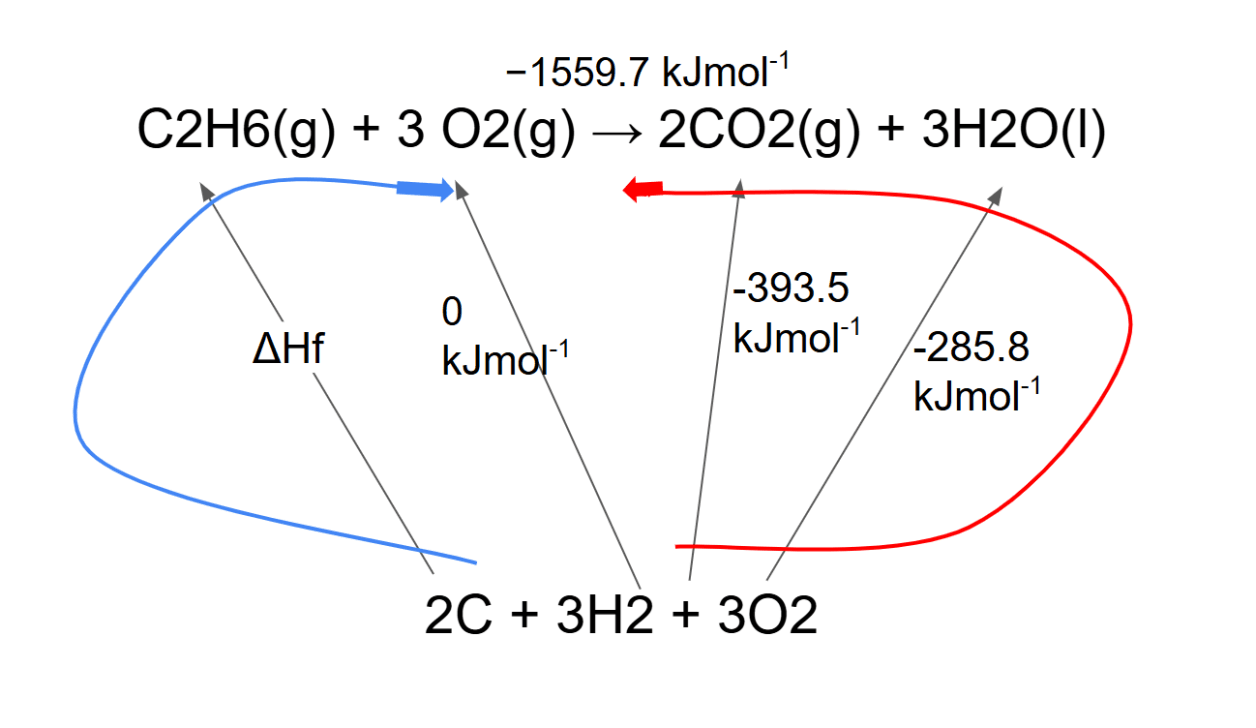
Hess' Law in enthalpy of formation
- We must find the enthalpy change of the reaction, ΔHr.
- The enthalpy change in the blue loop (2(-950.8) + ΔHr) must be the same as the enthalpy change in the red loop (-1130.7 + -393.5 + -285.8).
- 2(-950.8) + ΔHr = -1130.7 + -393.5 + -285.8
- Thus, we can calculate ΔHr, which is +91.6 kJ mol−1