Peptide bonds and amino acids
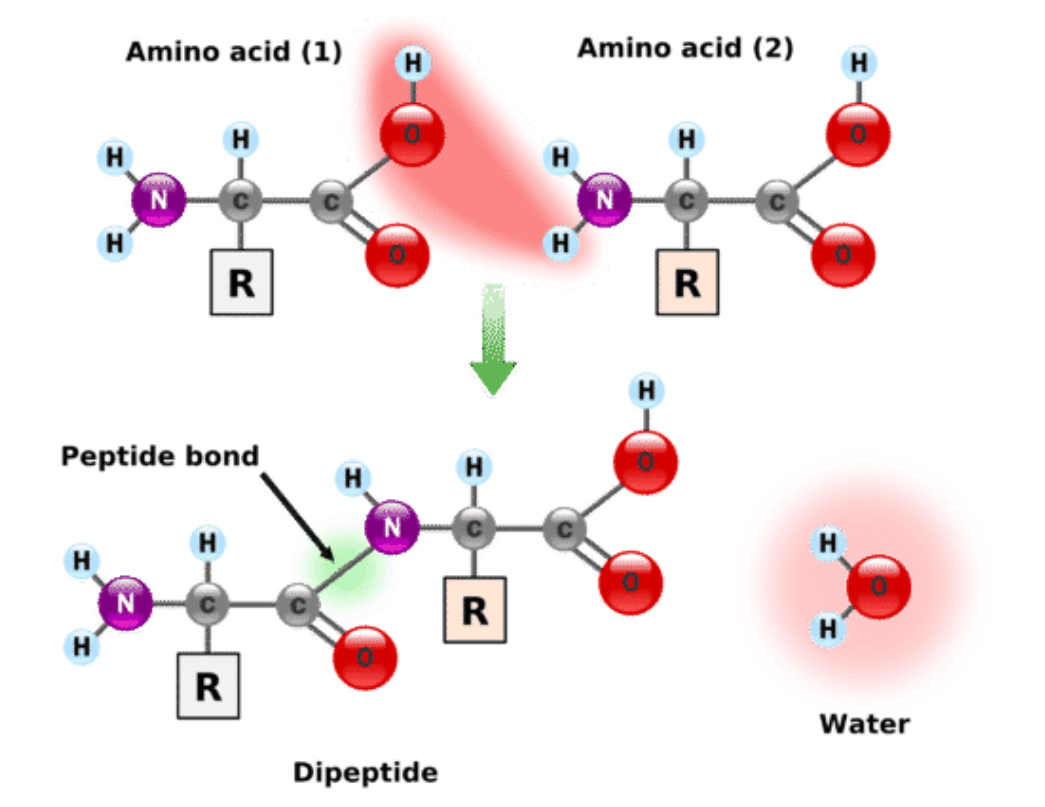
- Proteins are made from amino acids. All amino acids have a central carbon atom bonded to an amino group (-NH2) and a carboxylic acid group (-COOH).
- Amino acids are joined together by peptide bonds, which are covalent bonds.
- A peptide bond forms when one amino acid loses a hydroxyl (-OH) group from its carboxyl group and another loses a hydrogen atom from its amino group, resulting in the release of a water molecule.
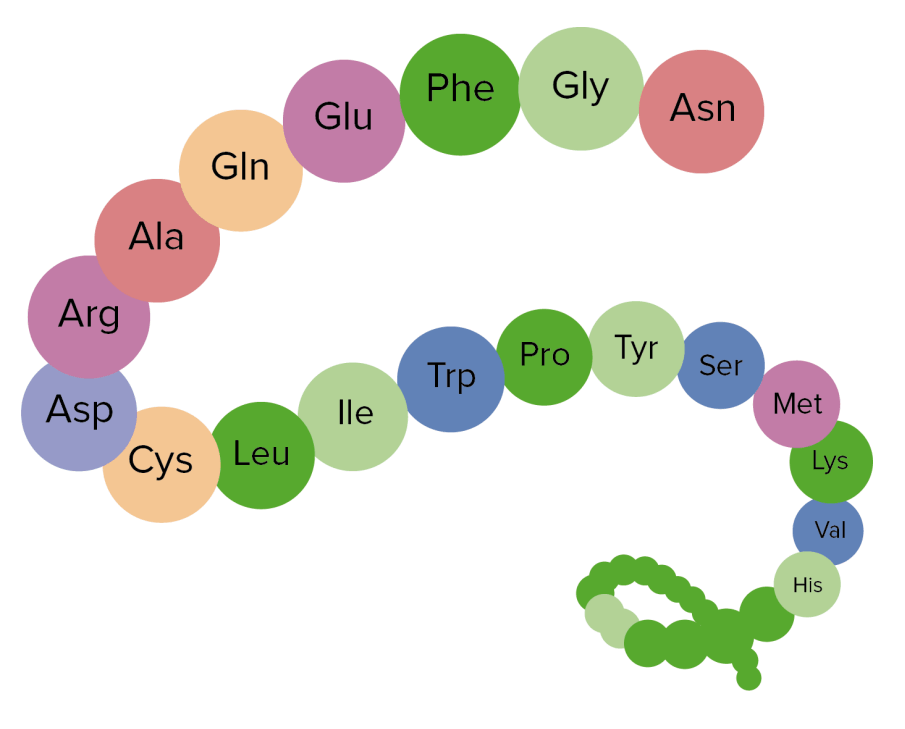
- Chains of amino acids linked by peptide bonds are called polypeptides, which form proteins.
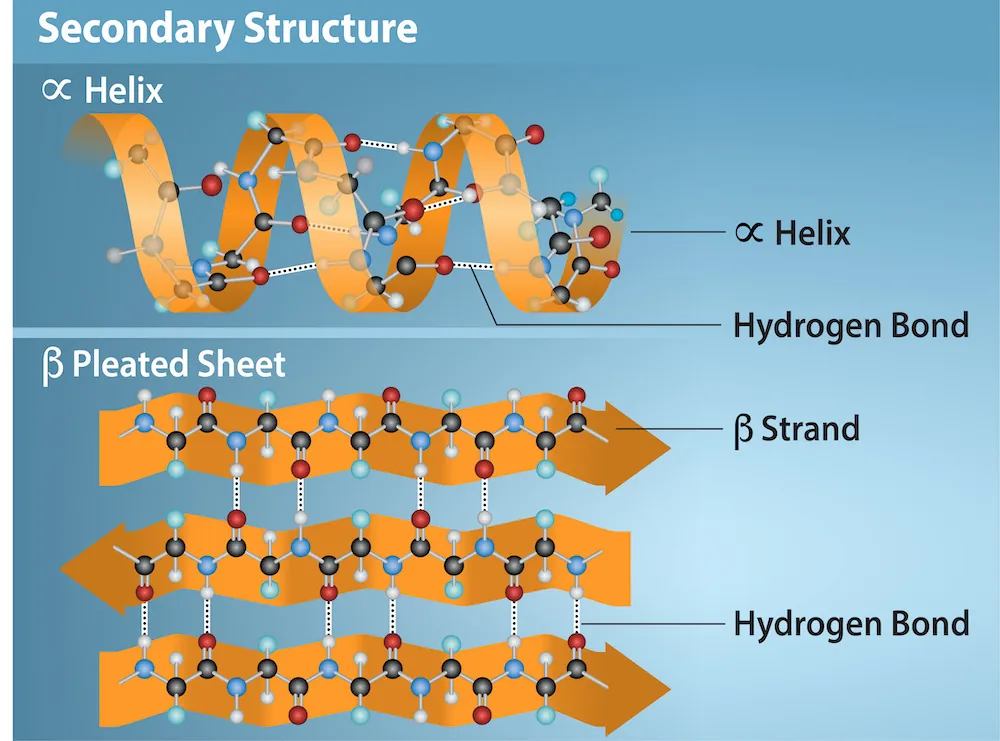
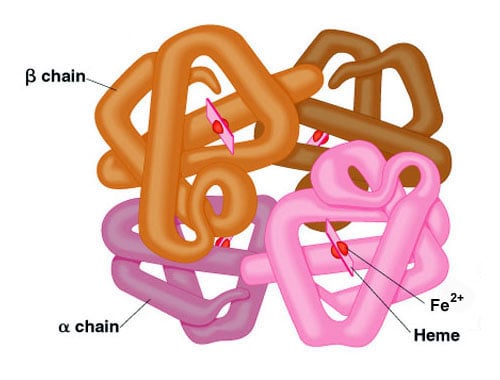
- Protein structure is split into four levels - primary, secondary, tertiary and quaternary.